North America Interventional Cardiology Market Analysis 2025–2033 | Reaching $11.98 Billion by 2033 DataM Intelligence
North America's Interventional Cardiology Market is set to grow from USD 7.04B in 2024 to USD 11.98B by 2033, driven by rising Heart Disease and Tech
The U.S. Interventional Cardiology Market is a major contributor to North America's $7.04B valuation in 2024, driven by rising heart disease rates and demand for minimally invasive procedures.”
AUSTIN, TX, UNITED STATES, June 13, 2025 /EINPresswire.com/ -- North America Interventional Cardiology Market: 2025 Outlook and Developments— DataM Intelligence
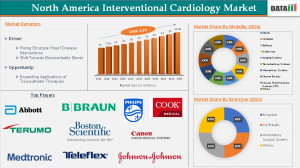
In 2025, the North American Interventional Cardiology Market Size was valued at USD 7.04 Billion and is expected to reach around USD 11.98 Billion by 2033, reflecting steady growth With a CAGR 6.2% at a strong compound annual rate over the forecast period 2025-2033
To Download Sample Report: https://datamintelligence.com/download-sample/north-america-interventional-cardiology-market
Latest Industry Developments
In December 2024, Terumo Interventional Systems (TIS) announced the U.S. commercial launch of its R2P NaviCross peripheral support catheter, marking another step in the expansion of its radial-to-peripheral (R2P) product line.
In October 2024, Medtronic plc received FDA approval for its Affera Mapping and Ablation System, including the Sphere-9 Catheter, designed for treating persistent atrial fibrillation (AFib) and performing RF ablation of CTI-dependent atrial flutter.
In July 2024, Teleflex gained FDA approval for its Ringer Perfusion Balloon Catheter (PBC) currently the only approved perfusion balloon available for Percutaneous Transluminal Coronary Angioplasty (PTCA) procedures in the U.S.
In March 2024, Boston Scientific received FDA clearance for its Agent Drug-Coated Balloon (DCB), developed to treat coronary in-stent restenosis (ISR) in patients suffering from coronary artery disease.
Regional Outlook
United States
In the U.S., interventional cardiology is undergoing rapid digitization. Robotic-assisted procedures, artificial intelligence in diagnostics, and remote surgical support tools are shaping the future of care. Key urban centers, such as New York, Boston, and San Francisco, are witnessing accelerated adoption of advanced tools like drug-eluting stents, intravascular ultrasound (IVUS), and optical coherence tomography (OCT).
Canada
Canada, though smaller in scale, shows remarkable progress.Government-driven initiatives promoting innovation in healthcare technology have accelerated the growth of regional R&D hubs. Additionally, cross-border collaborations with the U.S.-based healthcare firms are leading to faster adoption of novel cardiac care products in Canadian hospitals.
Key Companies Driving Innovation
Several major companies are shaping the future of interventional cardiology in North America:
Medtronic
Boston Scientific Corporation
Abbott.
Canon Medical Systems Corporation
Teleflex Incorporated.
B. Braun SE
Johnson & Johnson Services, Inc.
Koninklijke Philips N.V.
Cook Medical
Market Segmentation
By Modality: Stents, Drug-Eluting Stents, Bare-Metal Stents, Bio-Absorbable Stents, Catheters, Angiographic Catheters, Guiding Catheters, Balloon Catheters, Intravascular Ultrasound (IVUS) Catheters, Aspiration Catheters, Others, Balloons, Guidewires, Imaging Systems, Intravascular Ultrasound System (IUVS), Optical Coherence Tomography (OCT) Systems, Fractional Flow Reserve (FFR) Systems, Others, Thrombectomy Systems, Atherectomy Systems, Closure Devices, Chronic Total Occlusion Systems (CTO), Others
By End-User: Hospitals, CATH Labs, Ambulatory Surgical Centers, Others
Latest News of USA
In April 2025, a major milestone was achieved when a leading university hospital in Texas performed its first AI-assisted percutaneous coronary intervention (PCI) using a fully integrated robotic system. The system enabled precise stent placement with real-time feedback and predictive modeling, drastically reducing procedural errors.
Meanwhile, the FDA has recently approved a biodegradable stent system developed by a Silicon Valley med-tech firm. This device is designed to dissolve naturally in the body within 18 months, eliminating long-term complications often associated with metal stents. This innovation has caught the attention of cardiologists nationwide who seek to reduce repeat procedures and side effects.
Also making waves is the CardioTrack Initiative, launched in partnership between the American College of Cardiology and several top-tier device manufacturers. The program aims to install AI-enabled monitoring kiosks in rural communities to identify early signs of cardiac distress.
Latest News of Japan
Although the main focus is North America, Japan’s latest developments in interventional cardiology are creating ripple effects globally, including in North America through research collaboration and technology transfer.
In March 2025, Tokyo-based medical tech company Hirota Meditech unveiled a new bioresorbable valve replacement system, suitable for patients who are ineligible for open-heart surgery. The valve, designed for catheter-based implantation, is already gaining attention from global cardiac communities.
Additionally, Japan’s National Center for Global Health and Medicine announced successful clinical trials using a hybrid imaging system that combines intravascular ultrasound and AI-based blood flow analytics. This system has drastically improved the identification of vulnerable plaques a significant breakthrough for preventing heart attacks.
Japanese companies are also deepening ties with North American counterparts. For instance, a collaboration between Osaka University and a U.S. biotech firm is underway to co-develop smart microcatheters capable of delivering drugs precisely within coronary arteries.
Conclusion
The North American interventional cardiology market is in the midst of a revolution. Fueled by aging demographics, an increase in lifestyle diseases, and unparalleled advancements in technology, the region is setting new standards in cardiac care. From robotic procedures and biodegradable implants in the U.S. to precision imaging tools from Japan, the global ecosystem is closely interconnected.
As the market grows, so too does the promise of better, safer, and more efficient heart treatments making the future of cardiology both high-tech and highly human-focused.
Here are the Latest Experts Related Reports By DataM Intelligence
US Interventional Cardiology Market Size 2025-2033
Interventional Cardiology Market Size
Sai Kiran
DataM Intelligence 4Market Research
+1 877-441-4866
Sai.k@datamintelligence.com
Visit us on social media:
LinkedIn
X
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.