Self-Sampling HPV Test Kits Market Poised for 11.2% CAGR, Unlocking Growth for Manufacturers in Preventive Healthcare
Market surges from USD 410.6M in 2025 to USD 1,187M by 2035, fueled by home-based testing and global cervical cancer screening initiatives.
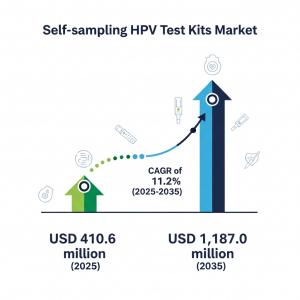
NEW YORK, DE, UNITED STATES, August 26, 2025 /EINPresswire.com/ -- The self-sampling HPV test kits market is entering an era of transformative growth, rising from USD 410.6 million in 2025 to USD 1,187.0 million by 2035, driven by a robust CAGR of 11.2%. For manufacturers, this trajectory is more than an opportunity—it’s a mandate to innovate, scale, and deliver solutions that address the evolving dynamics of women’s health diagnostics and at-home testing preferences.
At the heart of this surge lies a powerful global shift: early detection of cervical cancer through accessible and patient-friendly methods. Self-sampling HPV kits are emerging as game changers, breaking down barriers to screening and ensuring equitable healthcare access across diverse geographies.
Decentralized Healthcare: The Next Frontier in Diagnostics
As healthcare systems worldwide embrace decentralization, self-sampling HPV kits are bridging the gap between clinical accuracy and consumer convenience. By 2025, 28% of HPV testing in high-income countries will be conducted via self-collection, propelled by national screening mandates and technology-driven distribution models.
Digital health integration, coupled with mail-in testing frameworks, is extending the reach of screening programs into underserved communities. These solutions overcome traditional barriers like stigma, cost, and lack of infrastructure, creating a compelling value proposition for manufacturers ready to lead in this paradigm shift.
Click Here for More Information:- https://www.futuremarketinsights.com/reports/self-sampling-hpv-test-kits-market
Expanding Geographies, Expanding Possibilities
Emerging markets are becoming the epicenter of growth. India leads with a projected CAGR of 13.9%, followed by China at 12.2%, reflecting strong policy backing and rapid distribution expansion. Manufacturers investing in localized production, language-inclusive packaging, and region-specific partnerships stand to capture these fast-growing opportunities.
Europe and North America continue to show robust adoption, driven by regulatory support and telehealth integration. Meanwhile, nations like Japan, though growing at a slower pace, present unique prospects for culturally adaptive designs and privacy-centric features.
Dominant Segments Driving Demand
The market’s evolution is deeply tied to segmental leadership:
Vaginal Swab-Based Kits account for 49% of the market in 2025, winning consumer trust with ease of use and clinical reliability.
Cervical Cancer Screening dominates applications with an 88% share, positioning self-sampling as an indispensable tool in reducing global mortality from cervical cancer.
Hospitals hold 30% of the distribution channel share, leveraging physician trust to promote patient adoption, while online and direct-to-consumer platforms amplify convenience for the end user.
These dynamics highlight the strategic pathways manufacturers must prioritize to remain competitive—innovation, accessibility, and integration with healthcare ecosystems.
Technology and Quality: Key Catalysts for Growth
PCR-compatible kits are rewriting the rules of accuracy, with labs reporting 98% sensitivity for HPV-16 and HPV-18 detection from self-collected samples. Automated workflows now process up to 1,200 samples per day, reducing turnaround times and operational costs. For manufacturers, aligning products with such high-throughput capabilities ensures relevance in institutional procurement and public health partnerships.
Mail-in distribution is equally transformative, enabling 68% return compliance through prepaid logistics and SMS reminders. Dry swab technologies enhance DNA stability during transit, reinforcing the scalability of home-based models.
Global Health Initiatives: Fuel for Market Momentum
The World Health Organization’s cervical cancer elimination strategy and government-led screening mandates are accelerating the adoption of self-sampling kits. Public health programs across Latin America, South Asia, and Africa are allocating 5–7% of HPV prevention budgets to self-testing initiatives, signaling long-term procurement opportunities for manufacturers.
Collaborations with NGOs and integration into insurance schemes further extend reach, while regulatory approvals—such as Roche’s FDA clearance for its cobas self-collection system in 2024—underscore the clinical credibility of these innovations.
Get Sample Report: - https://www.futuremarketinsights.com/reports/sample/rep-gb-22485
Why Manufacturers Should Prioritize This Market Now
The convergence of clinical validation, consumer acceptance, and regulatory support makes self-sampling HPV kits a growth engine in diagnostics. Positioned within the USD 20 billion at-home testing market and the USD 30 billion women’s health diagnostics sector, these kits hold a transformative 6–8% share in cervical cancer screening.
Manufacturers who align product pipelines with affordability, digital integration, and high-throughput lab compatibility will not only secure market leadership but also shape the future of preventive healthcare.
Editor’s Note:
This press release highlights market potential and strategic growth pathways for manufacturers in the self-sampling HPV test kits segment.
Therapy Area Industry Analysis Reports:-
Cytomegalovirus Treatment Market
https://www.futuremarketinsights.com/reports/cytomegalovirus-treatment-market
Retinal Biologics Market
https://www.futuremarketinsights.com/reports/retinal-biologics-market
Isovaleric Acidemia Treatment Market
https://www.futuremarketinsights.com/reports/isovaleric-acidemia-treatment-market
Rahul Singh
Future Market Insights Inc.
+1 347-918-3531
email us here
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.