Global and European Inflammatory Bowel Disease (IBD) Treatment Market Outlook 2025–2035
Global IBD treatment market poised for steady growth by 2035, driven by biologics, novel therapies, and rising prevalence in Europe.
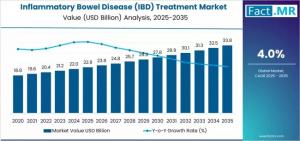
ROCKVILLE, MD, UNITED STATES, November 19, 2025 /EINPresswire.com/ -- The global inflammatory bowel disease (IBD) treatment market is set for steady growth from 2025 to 2035, driven by rising prevalence of Crohn’s disease and ulcerative colitis, adoption of advanced biologic therapies, and expanding access to novel treatment options. According to market insights, the global IBD treatment market is projected to increase from approximately USD 22.9 billion in 2025 to USD 33.8 billion by 2035, reflecting a compound annual growth rate (CAGR) of 4%. Europe represents a key region in this market, benefiting from increasing biosimilar adoption, strong healthcare infrastructure, and progressive reimbursement frameworks.Market Size and Growth
The IBD treatment market is experiencing robust expansion due to rising awareness, improved diagnostic capabilities, and the growing need for long-term disease management. Crohn’s disease dominates the market, accounting for over 60% of global demand, reflecting the extensive patient population requiring ongoing therapy and the availability of multiple treatment modalities, including TNF inhibitors, IL inhibitors, and JAK inhibitors. Europe’s market is poised for stable growth, supported by healthcare providers’ focus on cost-effective biologic adoption and patient access to specialized therapies.
To access the complete data tables and in-depth insights, request a Discount On The Report here: https://www.factmr.com/connectus/sample?flag=S&rep_id=11621
Key Growth Drivers
Rising disease prevalence: Both Crohn’s disease and ulcerative colitis are increasing globally, particularly in developed regions and newly industrialized countries, creating sustained demand for effective treatment options.
Adoption of advanced therapies: Biologic agents, JAK inhibitors, and emerging oral immunomodulators are becoming standard care, offering superior remission rates and improved patient outcomes compared to conventional treatments.
Regulatory approvals and innovation: Expanding approvals for novel therapeutic mechanisms, including S1P modulators and gut-selective biologics, are driving market adoption and enabling clinicians to tailor treatment plans.
Patient awareness and early intervention: Increasing understanding of IBD, along with improved diagnostic tools, is encouraging early and aggressive treatment strategies to prevent complications and reduce hospitalization.
European Market Insights
Europe demonstrates significant uptake of biosimilars due to cost-containment initiatives and hospital reimbursement schemes. Countries such as Germany, the UK, France, and the Nordic region are leading adoption of advanced therapies, including injectable biologics and oral small-molecule agents. Europe’s strong healthcare networks, academic medical centers, and specialty gastroenterology practices support the integration of innovative treatment protocols, while reimbursement variability and cost pressures remain challenges for high-end therapies.
Technology Trends and Competition
The European IBD treatment market features a competitive landscape with major players including AbbVie Inc., Takeda Pharmaceutical, Johnson & Johnson, Pfizer Inc., Eli Lilly, Biogen Inc., Novartis AG, UCB S.A., Celltrion Inc., and Merck & Co. Key trends include:
Expansion of biosimilar portfolios to improve affordability
Integration of JAK and IL inhibitors for personalized therapy
Development of oral immunomodulatory formulations for improved patient convenience
Digital health platforms supporting treatment monitoring and adherence
These trends enable healthcare providers to optimize treatment efficacy, reduce complications, and enhance patient quality of life.
Challenges
Despite positive growth, the market faces hurdles including high costs of biologic therapies, non-response in a subset of patients, and variability in reimbursement policies across European countries. Additionally, ensuring long-term remission and managing treatment adherence are ongoing challenges for healthcare providers.
Strategic Implications
For manufacturers: Focus on differentiated portfolios combining biologics, oral small molecules, and biosimilars. Investment in clinical trials and collaborations with healthcare institutions will strengthen market positioning.
For healthcare providers: Integrate advanced therapies, monitor patient outcomes using digital platforms, and adopt cost-effective biosimilars to manage rising IBD patient populations efficiently.
For investors: The market offers consistent growth opportunities, driven by rising disease prevalence, innovative therapy adoption, and European market stability.
Outlook Summary
From 2025 to 2035, the global IBD treatment market is expected to grow steadily, with Europe contributing significantly to overall demand. Rising prevalence, advanced biologic therapies, increased regulatory approvals, and awareness of early intervention will drive market expansion. While challenges such as treatment cost, non-response rates, and reimbursement variability persist, the market presents promising opportunities for manufacturers, healthcare providers, and investors focused on improving disease management and patient outcomes.
Purchase Full Report for Detailed Insights
For access to full forecasts, regional breakouts, company share analysis, and emerging trend assessments, you can purchase the complete report here: https://www.factmr.com/checkout/11621
Have a specific Requirements and Need Assistant on Report Pricing or Limited Budget please contact us – sales@factmr.com
To View Related Report:
Syndesmosis Repair System Market https://www.factmr.com/report/579/syndesmosis-repair-system-market
Next Generation Wound Closure Device Market https://www.factmr.com/report/582/next-generation-wound-closure-device-market
Paper-Based Diagnostic Test Kit Market https://www.factmr.com/report/583/paper-based-diagnostic-test-kits-market
Pacemaker/Defibrillator Lead Extraction Kits Market https://www.factmr.com/report/858/pacemaker-defibrillator-lead-extraction-kits-market
About Fact.MR
Fact.MR is a global market research and consulting firm, trusted by Fortune 500 companies and emerging businesses for reliable insights and strategic intelligence. With a presence across the U.S., UK, India, and Dubai, we deliver data-driven research and tailored consulting solutions across 30+ industries and 1,000+ markets. Backed by deep expertise and advanced analytics, Fact.MR helps organizations uncover opportunities, reduce risks, and make informed decisions for sustainable growth.
S. N. Jha
Fact.MR
+ +1 628-251-1583
email us here
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.